
|
Lorna Mills and Sally McKay
Digital Media Tree this blog's archive OVVLvverk Lorna Mills: Artworks / Persona Volare / contact Sally McKay: GIFS / cv and contact |
View current page
...more recent posts
 |
 |
 |
 | 
| 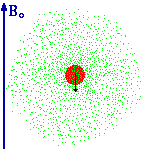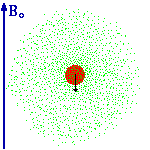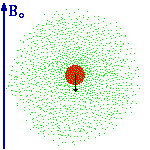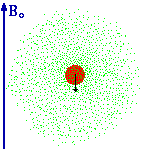 |
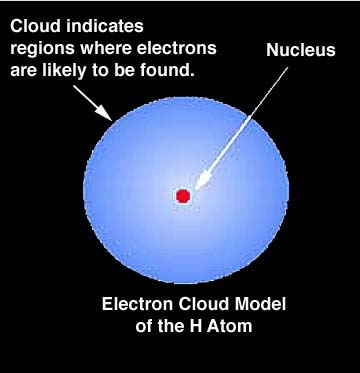
| From Thinkquest: "Now we can explain what the electron cloud is. It is the probability distribution of finding an electron around a nucleus. In places where the cloud thickens the probability of finding an electron grows. We can imagine "glancing" at an atom every second to find where we can see an electron. It will show that in most cases we see an electron in the place where the Schroedinger function defines the probability of finding it as high. Very rarely we would see an electron in the places where the probability is small, and never in places where there is nil probability calculated with Y2 function (That is for example inside the nucleus.). Yet let's take a piece of paper with the nucleus marked on it and mark with a point each electron we have noticed. After a while we will get a picture of an electron cloud." | ||